Becky Lamis, PharmD, FISMP
Manager, Med Safety Board
The Institute for Safe Medication Practices (ISMP) received a safety concern from clinicians through its national error reporting program regarding the packaging of an enteral/oral probiotic. ISMP, which is Med Safety Board’s parent organization and an ECRI affiliate, subsequently communicated this risk to the healthcare community in a February 2023 article titled Safety issue with probiotic packaging.
The probiotic is intended for administration orally or via enteral feeding tubes to babies in the neonatal intensive care unit; however, it is supplied in a vial that appears similar to those used for parenteral injection (Figure 1). The concern is that practitioners may mistake the product for a parenteral medication vial and withdraw a dose using a parenteral syringe and needle. This could then lead to a wrong-route error, with the probiotic being administered intravenously (IV), resulting in possible patient harm in an already vulnerable patient population.

Figure 1. Top of vial with cap removed and metal ferrule partially peeled away from the rubber stopper.
As noted by ISMP, “Whenever a substance meant for one route is placed in packaging more typically used for another route, the chance of administering the substance by the wrong route is increased.”
Additionally, the route statement, “For Enteral or Oral Use,” on the probiotic is printed in a small font size and not prominently placed on the vial; practitioners may easily miss this warning as they must turn the vial to read it on the back side of the label (Figure 2).

Figure 2. The “For Enteral or Oral Use” warning is only visible if a practitioner turns the vial to read the back of the label.
Medication products packaged in containers typically used for drugs that are administered via a different route is not a new issue. ISMP has repeatedly warned about this safety concern in the ISMP Medication Safety Alert! Acute Care newsletter. ISMP has reported on several products supplied by either pharmaceutical companies or 503B outsourcing facilities that have the potential to result in or have resulted in wrong-route errors and patient harm, including the following:
- Topical hemostatic agent supplied in a carton with Luer-tip (parenteral) syringes that has been inadvertently administered IV
- Oral imaging agent provided in a parenteral vial that could be accidentally given IV
- Topical analgesic prepared in a parenteral syringe that could be mistaken for a parenteral injection
- Ear drops manufactured in an ophthalmic container that have been instilled erroneously in the eye
- Orally inhaled product packaged in capsule form that has been accidentally swallowed instead of inhaled using a device
- Concentrated electrolytes that need to be diluted first presented in a ready-to-administer container (e.g., bag, prefilled syringe) that could be erroneously infused directly to the patient
Recommendations
As recommended by the US Food and Drug Administration’s (FDA) guidance for industry titled Safety Considerations for Product Design to Minimize Medication Errors:
“The best container closure designs are those that do not require extensive end-user training and that make sense for the dose, route, and method of administration.” The FDA further notes that “Drug products should not be packaged in a container/closure system that implies or affords a route of administration other than the route intended, unless there are no other options available, because this practice has led to wrong routes of administration.”
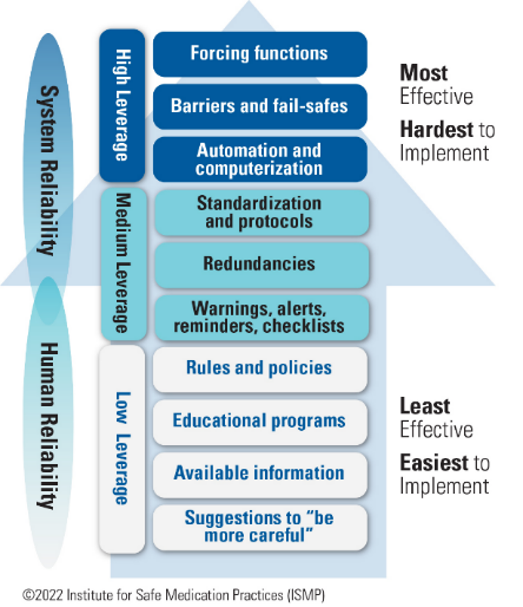
Designing a container closure that is intuitive to clinicians and allows them to correctly recognize the product’s route of administration is a high-leverage strategy (Figure 3) and will have a greater impact on safety than simply relying on practitioners to heed a warning on the product label; high-leverage strategies make it harder for practitioners to do their job wrong and easier for them to do it right.
To further minimize the risk of wrong-route errors, the following labeling strategies should also be implemented:
- Present the route of administration on the principal display panel, along with other critical information (e.g., drug name, concentration/strength), so that end users do not need to turn or rotate the container; if the warning is not within an individual’s direct line of sight, then it may be overlooked.
- Prominently communicate the route of administration by maximizing the font size used, ensuring the color contrast between the background and text makes the message easily readable, and using boxing or color to highlight this important information if needed based on the risks.
- Use positive statements, such as “For Topical Use Only,” when listing the route of administration; the “not” in negative statements, such as “Not for Injection,” can easily be missed.
- Consider other strategies to help visually convey the correct route of administration (e.g., using a graphic of an eye on the label for an eye drop), other means of modifying the container to limit practitioners’ ability to administer it via the wrong route, and/or other “out of the box” ideas for readily capturing end users’ attention.
Many of the above recommendations can also be found in the FDA’s guidance for industry titled Safety Considerations for Container Labels and Carton Labeling Design to Minimize Medication Errors.
As early as possible in the product development process, pharmaceutical manufacturers and 503B outsourcing facilities should consider the potential for error by proactively assessing the design of their new product using failure mode and effects analysis (FMEA). During the FMEA process, potential error scenarios and their outcomes, along with associated mitigation strategies, should be identified for each relevant step in the medication-use process. Employing the use of proactive risk assessments through FMEA and simulated use testing at the earliest stages of product design is recommended by the FDA in their Safety Considerations for Product Design to Minimize Medication Errors guidance for industry. Identifying issues early in the process allows for more time to modify the container design, if needed, and to implement high-leverage safety strategies, minimizing the risk of patient harm while also limiting delays in product launch.
Learn more about Med Safety Board’s risk assessment services that incorporate FMEA and our human factors offerings, including usability testing. We can assist you throughout the product development process to identify potential issues, including wrong-route errors, and strategies for mitigating those risks. Contact Med Safety Board for more information and to speak with our experts.