Becky Lamis, PharmD, FISMP
Manager, Med Safety Board
Earlier this year, Med Safety Board’s parent organization and ECRI-affiliate, the Institute for Safe Medication Practices (ISMP), published an article entitled, Latent and active failures perfectly align to allow a preventable adverse event to reach a patient, which described the multiple failures that led to a patient erroneously receiving an intravenous (IV) infusion of midazolam instead of magnesium sulfate.
While multiple latent and active failures were identified as contributing factors in this event, one key latent failure, or system issue was identified; both the midazolam and magnesium sulfate premixed products appeared very similar once the IV bags were removed from their overwraps. This case illustrates the importance of ensuring that overwrap labeling is well-differentiated between products within a company’s line and that bag labels are also sufficiently distinct from one another. Making this visual identification can help minimize the risk of potentially harmful mix-ups.
Error Event
In the reported event, the nurse had pulled what was believed to be two 100 mL bags of magnesium sulfate 1 g from an automated dispensing cabinet (ADC) for a patient who had been prescribed 2 g of IV magnesium sulfate for hypomagnesemia. The nurse removed the aluminum overwraps from each bag and hung them on the patient’s IV pole. After the first bag had finished infusing, the nurse replaced the empty bag with the second bag. Instead of barcode scanning the new bag, though, the nurse scanned the empty bag that had already been infused. While the first bag did contain magnesium sulfate 1 g, unbeknownst to the nurse, there had been a pharmacy dispensing error when refilling the ADC and the second bag actually contained midazolam 100 mg in 100 mL, not magnesium.
As a result of the error, the patient experienced respiratory depression, which the clinical team attributed to progression of the patient’s illness. The patient was in palliative care, and had a “do not resuscitate” order, so aggressive measures were not used. When removing the bags from the IV pole, the nurse identified the error, at which point flumazenil was ordered, but the patient later died that same morning. However, the accidental administration of midazolam was not believed to be a proximal cause of death in this case.
Error Analysis
The midazolam 100 mg and magnesium sulfate 1 g premixed products involved in this reported event were both manufactured by the same pharmaceutical company and supplied in 100 mL bags within aluminum overwraps. While the overwrap labels utilize different design elements that may help to differentiate these products (Figure 1), after these IV products are removed from their overwraps, the labels printed on the bags are much more similar and difficult to tell apart (Figure 2). Also, the poor color contrast between the light orange font used to print the drug name and strength on the clear background for both bags makes this critical information difficult to read, further limiting the ability of end users to easily distinguish between these two products.
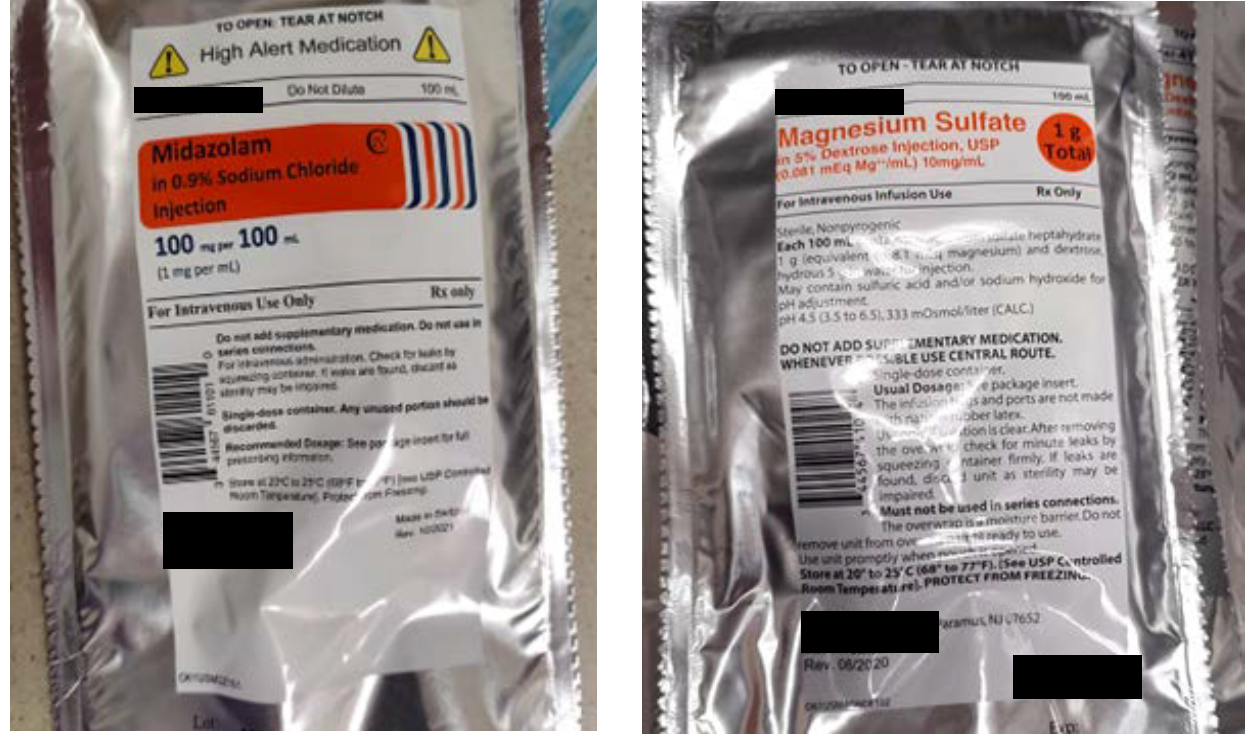
Figure 1. The label on the midazolam 100 mg per 100 mL overwrap (left) compared to the magnesium sulfate 1 g per 100 mL overwrap label (right).
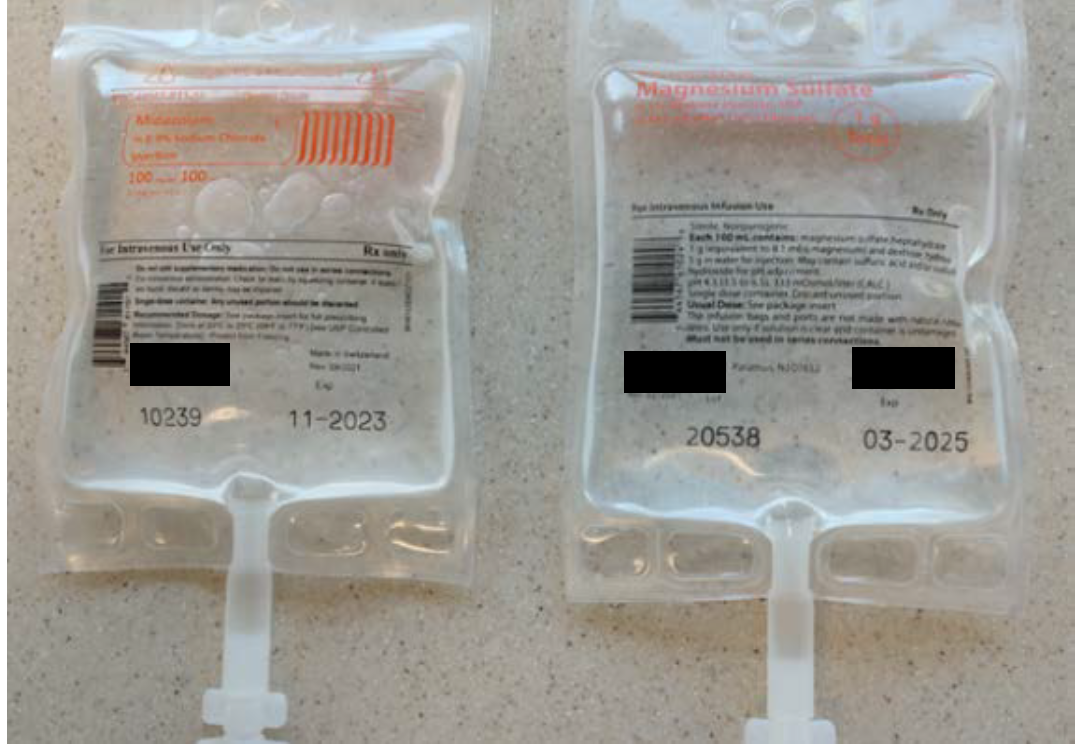
Figure 2. Once removed from their overwraps, midazolam 100 mg per 100 mL (left) and magnesium sulfate 1 g per 100 mL (right) bags have similar labels.
This case demonstrates that it is crucial to ensure that both the overwrap labeling and container label are well-differentiated between products. Doing so can help minimize the risk of mix-ups during the manufacturing process and again in the pharmacy, when dispensing products to patient care units, and prior to administration. Additionally, this error demonstrates that manufacturers cannot rely on barcode scanning being used to catch mix-ups with their products because workarounds or process flaws might exist at various points in the system.
Recommendations
To minimize the risk of an event similar to this one and the subsequent possibility of patient harm, pharmaceutical manufacturers should proactively evaluate existing labels for look-alike products and redesign if needed; a company’s product label, including the carton and overwrap labeling and the container label, should be distinct from their other product labels. While some life sciences organizations may want to create a “brand look” for their medication labels within the same product line, this can lead to unsafe labels. The use of different colors and other design elements, such as boxing and reverse print, can help make product labels appear more distinct and easily identifiable by end users.
As recommended by the US Food and Drug Administration’s (FDA) guidance for industry entitled, Safety Considerations for Container Labels and Carton Labeling Design to Minimize Medication Errors,
“Sponsors should create a container label and carton labeling design that is sufficiently distinct from that of their other products so that the end user is able to correctly identify, select, dispense, and administer the appropriate medication, strength, and dose.” Additionally, FDA states that “Sponsors should consider whether the use of corporate trade dress could make it difficult for end users to distinguish between different medications or different strengths of the same medication within their product lines, and should ensure there are means to distinguish the container labels and carton labeling of multiple products.”
In addition, the following recommendations also can help enhance product label clarity and therefore, reduce error risk and patient harm:
- Evaluate the appearance of carton or overwrap labeling and container labels when placed on the actual product packaging or container. For example, an IV bag label printed on a clear background will appear different from when it is viewed in an electronic format with a white background.
- Maximize the color contrast ratio between the background color and the text on the label, including text printed on a clear bag, to ensure the content is easily readable.
- Utilize a white background for bag labels, especially for the critical information, including the drug name, concentration/strength, route, and warnings, for improved readability; this will allow for the use of more color design options to differentiate products.
- Add a label with the critical information, including the drug name, concentration/strength, route, and warnings, to the back side of aluminum overwrap and possibly the bag if a white label background is used, so that the double-sided printing doesn’t result in overlap with other text on the transparent bag. Repeating this information on both sides can aid in visualization and correct identification of the product no matter which way it is set down or turned.
Learn more about Med Safety Board’s label review and design services and how we can assist you with evaluating your product labels or designing them to minimize the potential for product mix-ups and resulting patient harm. Or contact Med Safety Board for more information and to speak with our experts.